This site is intended for
US residents only.
Please confirm your location.
GELCLAIR® is a prescription gel that works locally to gently soothe the pain of oral mucositis for several hours at a time
Indications: GELCLAIR® has a mechanical action indicated for the management of pain and relief of pain by adhering to the mucosal surface of the mouth, soothing oral lesions of various etiologies, including oral mucositis/stomatitis (may be caused by chemotherapy or radiation therapy), irritation due to oral surgery, traumatic ulcers caused by braces or ill-fitting dentures, or disease. Also, indicated for diffuse aphthous ulcers.

GELCLAIR® contains ingredients that soothe, coat, and hydrate the lining of the mouth, and may reduce the severity of oral mucositis and associated symptoms like pain and problems eating normally. Because GELCLAIR® doesn’t contain alcohol or numbing agents, there should be no stinging, drying, or numbing when you use it for the treatment of mouth ulcers and mouth pain.
GELCLAIR® works rapidly; in clinical trials, patients experienced a significant reduction in pain following the first application. A majority of patients reported pain relief and the ability to eat and drink more comfortably, with improvements in the severity of oral mucositis within 2 to 3 days.
GELCLAIR® comes in a portable gel packet and may be diluted with water or used without dilution.
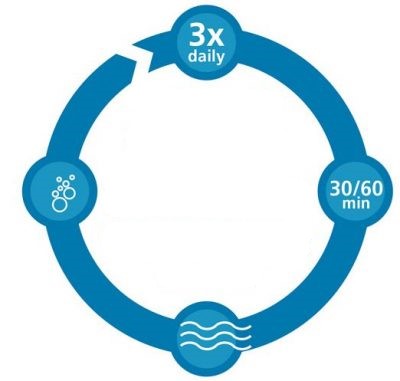
Follow these steps for pain relief of oral mucositis
INDICATIONS
GELCLAIR® has a mechanical action indicated for the management of pain and relief of pain by adhering to the mucosal surface of the mouth, soothing oral lesions of various etiologies, including oral mucositis/stomatitis (may be caused by chemotherapy or radiation therapy), irritation due to oral surgery, traumatic ulcers caused by braces or ill-fitting dentures, or disease. Also, indicated for diffuse aphthous ulcers.
IMPORTANT SAFETY INFORMATION
You are encouraged to report negative side effects of
prescription medical products to the FDA.
Visit
www.fda.gov/safety/medwatch, call 1-855-273-0468 or fill-in the form at this
link.
Please see full Prescribing Information.